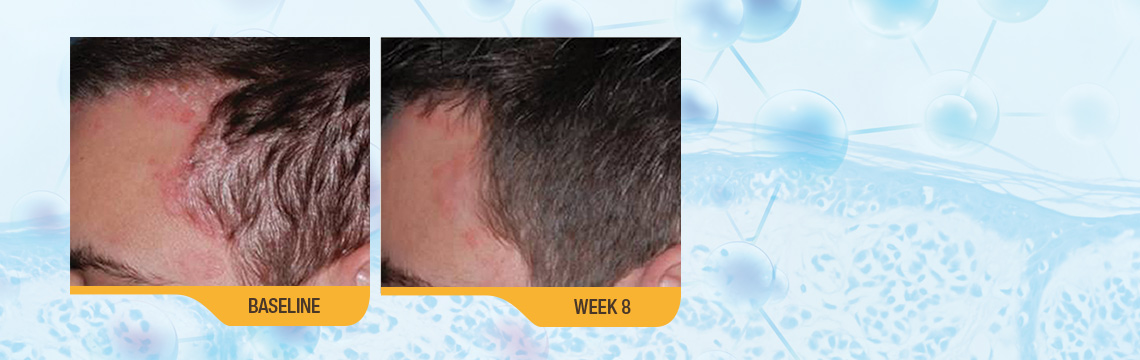
For your patients with scalp psoriasis
In a Phase III clinical study of moderate–severity scalp psoriasis, significantly more patients treated with SORILUX Foam achieved treatment success compared with vehicle foam.1
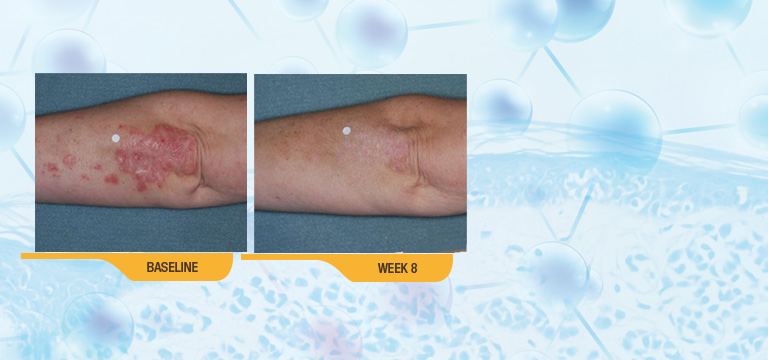
For your patients with body psoriasis
In two 8-week, Phase III studies, SORILUX Foam was shown to be safe and effective for treating mild to moderate plaque psoriasis of the body.2,3
Give your patients the only single-agent calcipotriene therapy available in an elegant foam formulation
SORILUX Foam delivers effective, steroid–free treatment for scalp and body psoriasis.
- Penetrates the skin barrier to deliver the molecule into the epidermis and dermis4
- Aqueous-based emulsion that is easy to apply to both hair-bearing and non–hair-bearing areas5
- Absorbs quickly without greasiness or residue4,5
- No ethanol, preservatives, or fragrances
- The contribution to efficacy of individual components of the vehicle has not been established
Most insured, eligible patients will pay $0 for a SORILUX Foam prescription.
References: 1. Feldman SR, Eastman WJ, Brundage T, Mills M. A multicenter, randomized, double-blind study of the efficacy and safety of calcipotriene foam, 0.005%, vs vehicle foam in the treatment of plaque-type psoriasis of the scalp. J Drugs Dermatol. 2013;12(3):300-306. 2. Feldman SR, Matheson R, Bruce S, et al; U0267-301 & 302 Study Investigators. Efficacy and safety of calcipotriene 0.005% foam for the treatment of plaque-type psoriasis: results of two multicenter, randomized, double-blind, vehicle-controlled, phase III clinical trials. Am J Clin Dermatol. 2012;13(4):261-271. 3. SORILUX Foam [package insert]. Greenville, NC: Mayne Pharma; 2019. 4. Data on file. Greenville, NC; Mayne Pharma LLC. 5. Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(2):AB50.